COVID-19 booster shot debate has medical experts divided
September 26, 2021
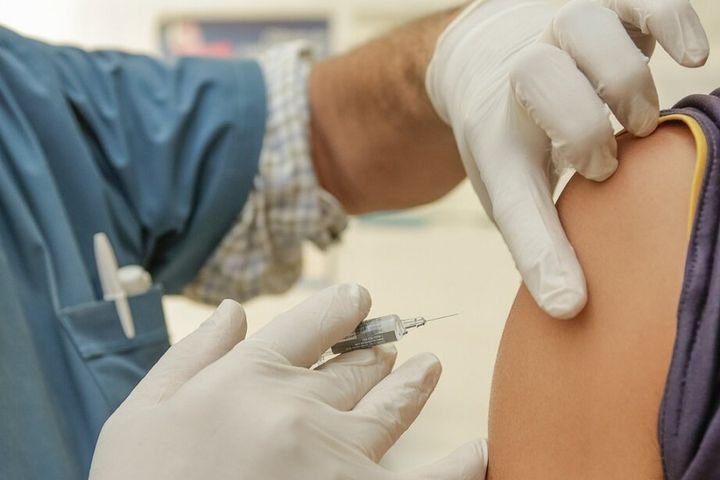
The U.S. Food and Drug Administration advisory panel recommended emergency use authorization for the Pfizer-BioNTech booster shot for Americans 65 and older and people that are at high risk of serious complications, such as immunocompromised individuals.
The FDA, however, rejected extending booster shot availability to people under 65 years old. “After seven hours of deliberation, members of the Vaccines and Related Biological Products Advisory Committee voted 16 to 2 against a proposal to administer a third dose of the vaccine developed by Pfizer and BioNTech to individuals 16 years and older,” Stat News said. The unanimous decision was made on Sept. 17.
Comparatively, the vote to grant boosters to older Americans and those at risk of severe COVID- 19 infection was 18 to 0.
President Joe Biden planned to get boosters to eligible Americans this fall, during the week of Sept. 20. Although Biden selected this tentative date, his decision to push boosters pended on the FDA and Center for Disease Control and Prevention’s approval, The White House said. This was to determine the effectiveness and safety of the boosters.
The timing of the booster shots was scheduled to provide additional protection to those that received their first doses of the COVID-19 vaccine back around late December and early January because their immunity wanes after about six months. “The data in this report demonstrate that [the Pfizer vaccine] prevents COVID-19 effectively for up to 6 months post-dose 2 across diverse populations, despite the emergence of SARS-CoV-2 variants,” Pfizer stated in a study.
Among those that were first to receive a COVID-19 vaccination were residents in health care facilities, health care providers and older adults.
The debate over whether to allow broader use of boosters has been a back and forth between experts. Some think that distributing boosters to every eligible adult would be premature without proper data to support the decision. And some don’t see a need for boosters because the fully vaccinated still have protection from infection.
“Overall data indicate that currently U.S.-licensed or authorized COVID-19 vaccines still afford protection against severe COVID-19 disease and death in the United States,” FDA staff members said, according to Reuters.
The World Health Organization was strongly opposed to booster shots because of a vaccine equity issue. Officials called for wealthy nations to stop distributing the boosters and encouraged them to shift their focus to making shots widely accessible for countries with lagging rates, CNBC said.
Within America, there is still an issue of vaccine equity for those that got the Johnson & Johnson vaccine. The CDC mentioned that there isn’t enough data to currently support someone who got the J&J vaccine getting an mRNA vaccine booster dose. This means that more studies need to be done and that these Americans may have to wait for J&J to release their own booster shot.
On the other hand, many have sided with Biden and endorsed the booster shots, including FDA Acting Commissioner Janet Woodcock and CDC Director Rochelle Walensky, ABC News said. An increase in hospitalizations and deaths due to the Delta variant has also been cited as a reason to support boosters. “With the Delta variant, public health experts are starting to see reduced protection against mild and moderate disease,” the CDC said.
“Confronting that onslaught with an equally massive immune response, which, it seems, can be generated by a booster dose of the original vaccine, is sufficient to keep Delta at bay—and keeping any virus that does get through from causing severe disease,” Time said.
According to ABC News, Pfizer’s data suggests boosters give protection against breakthrough infection. They assured people their booster was safe because, during the clinical trials, there were no safety concerns.