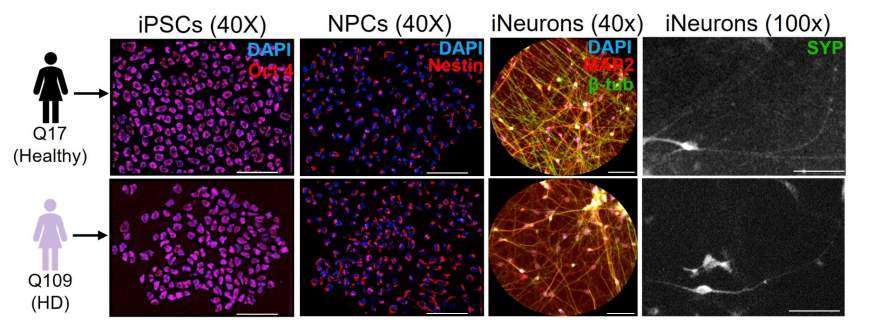
Researchers at the University at Buffalo found two key enzymes that could alter our approach to treating Huntington’s disease, a devastating neurodegenerative disorder characterized by neuronal loss and severe cognitive decline. There are two signaling proteins, glycogen synthase kinase-3 beta, GSK3β and extracellular signal-regulated kinase 1, ERK1, which play opposite roles in disease progression. HD is caused by a genetic mutation that expands a region known as polyQ within the Huntington protein, HTT. This mutation disrupts the normal function of HTT, leading to axonal degeneration, impaired neuronal function and cell death.
Traditionally, research has focused most on the neuronal consequences of expansions. However, this study shifts attention toward the cellular pathways that control HTT movement within neurons, especially the kinases, which are proteins that modify other proteins. Through detailed proteomic analyses and experiments in fruit fly models, the team found a dramatic shift in kinase activity in HD-affected neurons. Specifically, that elevated activity of GSK3β worsened disease symptoms. Conversely, increased ERK1 activity was protective, reducing these blockages and even mitigating cell death.
“With these findings, we propose that ERK1 may protect neurons in the face of Huntington’s disease, while GSK3ß may exacerbate Huntington’s disease,” senior author Shermali Gunawardena said. “Therapeutics may one day be able to target these signaling proteins in different ways — inhibiting GSK3ß and boosting ERK1 — to treat this severe and fatal neurological disorder.” The researchers validated their findings by genetically manipulating these enzymes in fruit fly larvae expressing the mutated HTT protein. Larvae treated to inhibit GSK3β showed improved axonal transport, reduced neuronal death and better motor function.
In contrast, inhibition of ERK1 produced the opposite effect, worsening neuronal blockages and cell death. “So, while GSK3ß typically plays a positive role in neuronal function, it seems it may actually make a bad situation worse when faced with a mutant HTT,” Gunawardena noted. The paradoxical role of GSK3β presents both a new therapeutic challenge and opportunity. Therapeutics targeting this enzyme may need careful calibration to avoid unintended side effects.
Conversely, boosting ERK1 activity could offer a safer approach to therapy, provided it does not disrupt other critical cellular functions. “The level of ERK1 is clearly important for Huntington’s disease, but whether it’s actually modulating the mutant HTT is unclear,” first author Thomas Krzystek explained. “Either way, the signaling from this ERK1 pathway is neuroprotective in the context of Huntington’s disease.” Ultimately, these results provide a dual roadmap for therapeutic development, not only highlighting potential new drug targets but also underscoring the complex molecular dance within neurons in HD. Researchers may soon be able to fundamentally alter disease progression.