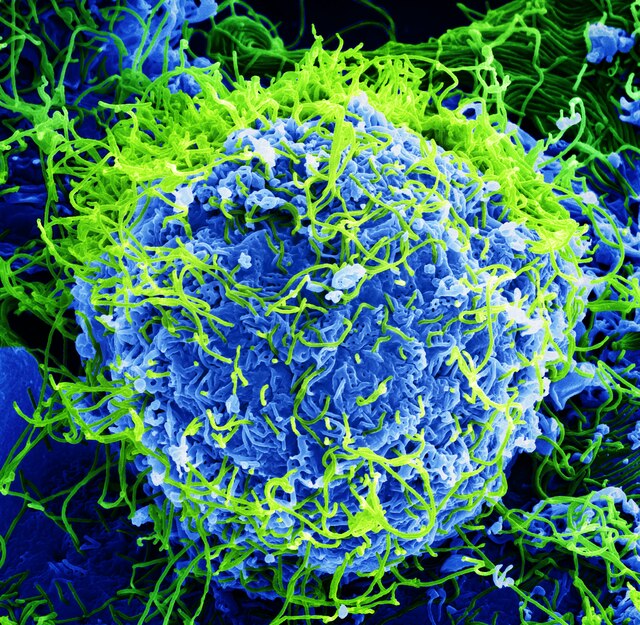
COF-999, a covalent organic framework, is designed to capture CO2 from open air using its intricate porous structure. Under a microscope, COF-999 appears as tiny particles filled with millions of microscopic holes, each tailored to selectively absorb carbon dioxide molecules while letting other gases pass through.
According to Zihui Zhou, a UC Berkeley materials chemist who co-led the research, the key to COF-999’s effectiveness lies in these pores, which are lined with compounds called amines. Due to their basicity, amines are chemically attracted to the acidic nature of CO2 molecules, allowing for a stable capture process.
Once CO2 is captured, it can be released from the powder by heating it to a modest temperature of around 140 degrees Fahrenheit—much lower than many other carbon capture materials require.
This low release temperature reduces the energy costs associated with capturing and storing CO2. After the captured CO2 is released, it can then be securely stored, typically by injecting it deep underground, where it no longer contributes to atmospheric warming.
The release process also allows COF-999 to reset for reuse, making it an efficient option for continuous air capture.
COF-999 is notably different from existing carbon capture technologies. Traditional carbon capture often relies on liquid solvents or solid sorbents, which require large-scale facilities, complex chemical processes or high temperatures to release the captured CO2. These existing methods are often energy-intensive and costly, limiting their practicality for widespread use.
In contrast, COF-999’s lightweight and highly porous structure allows it to capture CO2 up to 10 times faster than many of its predecessors.
Its polyamine-coated pores provide more surface area for CO2 absorption, enhancing the efficiency of capture. According to Omar Yaghi, a reticular chemist and senior author of the study, COF-999’s durability is another critical advantage.
It can go through hundreds and potentially thousands of capture-release cycles without losing effectiveness. Yaghi noted that this longevity could make COF-999 ideal for large-scale applications in direct air capture facilities, reducing the need for frequent replacement and lowering operational costs.
Another difference is that COF-999 operates effectively in ambient outdoor conditions, unlike some existing carbon capture materials that require controlled environments. In a test conducted in open-air conditions at Berkeley, COF-999 performed consistently for more than 100 cycles. The material fully retained its carbon capture ability, demonstrating its robustness and potential for real-world applications.
This adaptability to natural conditions means COF-999 could be implemented in a range of settings, from urban areas to industrial sites, providing flexibility for carbon capture efforts across diverse environments.
Effective carbon capture is becoming increasingly essential in the global effort to combat climate change. Scientists warn that even if carbon emissions were halted today, atmospheric CO2 would remain at dangerously high levels, continuing to warm the planet for decades.
By actively removing CO2 from the atmosphere, direct air capture technologies like COF-999 could help offset unavoidable emissions from sectors like heavy industry, which are challenging to decarbonize completely.
Keeping CO2 levels below 450 parts per million is considered critical to limiting global warming to below 2 degrees Celsius, a threshold widely agreed upon by scientists to prevent the most severe impacts of climate change. Current CO2 levels hover around 423 ppm, and without intervention, these levels are likely to continue rising, threatening ecosystems, weather stability and public health.
Carbon capture materials like COF-999 could play a crucial role in achieving the necessary reduction in atmospheric CO2. While carbon capture is not a complete solution to climate change, experts agree it is an essential component of any effective climate strategy.
Klaus Lackner, a director at Arizona State University’s Center for Negative Carbon Emissions, said that materials like COF-999 are “opening a door into a new family of approaches” that could make direct air capture more viable on a global scale. However, Lackner also emphasizes that for carbon capture to make a meaningful impact, the process needs to become 10 times cheaper than current options. Advances in materials like COF-999 may help bring costs down, making large-scale deployment more feasible.
Yaghi and his team are optimistic about the material’s potential to be integrated into direct air capture plants within two years, though the exact costs are still being evaluated. The material’s resilience, efficiency and ease of use could make it an attractive option for governments and industries looking to reduce their carbon footprint and make meaningful strides toward climate goals.
While carbon capture alone cannot solve climate change, COF-999 represents a significant step forward in making atmospheric CO2 reduction feasible, scalable and economically viable. As part of a larger strategy that includes renewable energy, conservation and emission reductions, this promising technology could help support a cleaner, cooler future for the planet.